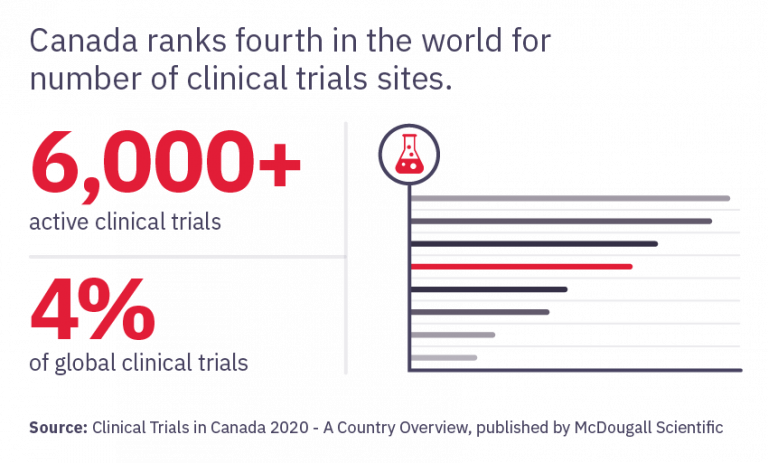
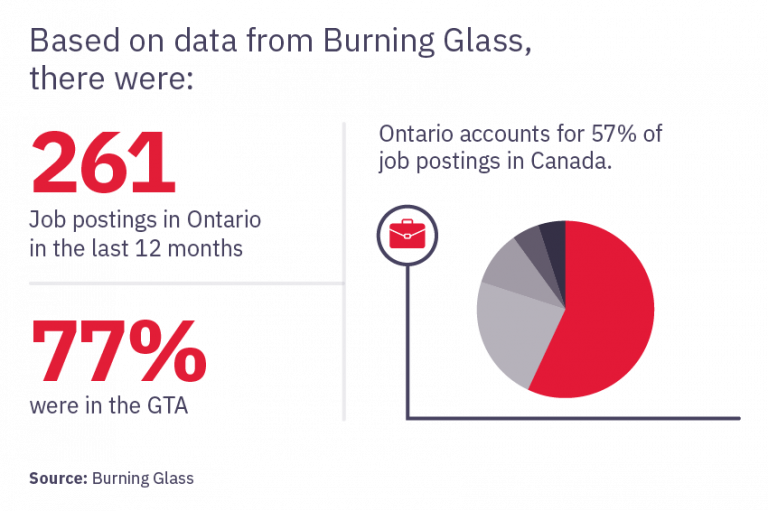
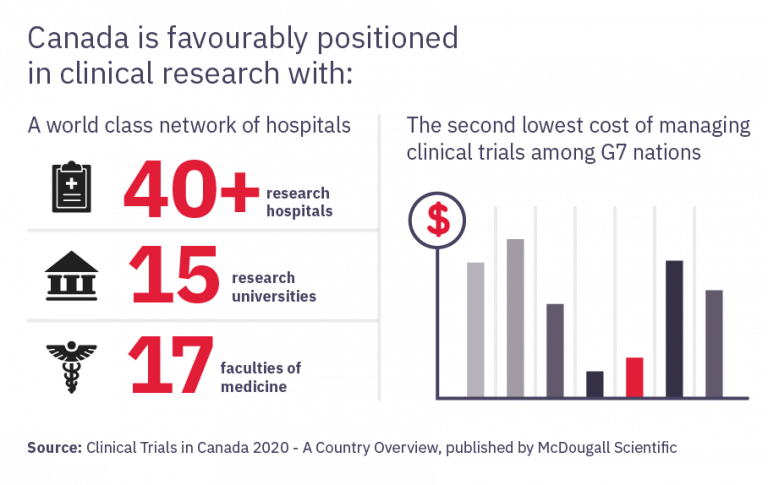
Become an essential member of the clinical research process by learning how to protect patient safety, ensure trial integrity, and manage adherence to research ethics, best practices, and regulations.
What You Will Learn
In our part-time Certificate in Clinical Research, you’ll prepare for a career on the cutting edge of this field with instruction from leaders in clinical research who bring a practical perspective to the curriculum. This program will help you:
- Understand the stages in setting up clinical trials
- Plan, manage, and monitor clinical research and trials
- Adhere to good clinical practice including patient consent, privacy, and data integrity protocols
- Abide by regulations and legislation to ensure that trials are conducted ethically while upholding scientific research principles
- Demonstrate accuracy and reliability in data collection, management, and analysis
Program Benefits
- Practice applying clinical trial procedures, regulations, and best practices through experiential assignments, projects, and case studies
- Advance through the program with the same cohort of peers, allowing you to develop a strong professional network
- Apply your learnings in an applied clinical research management simulation
- Balance your commitments with our blended study option which combines live classes with asynchronous online learning
- Complete the program faster by earning your certificate in only nine months
Webinars
- Careers in Clinical Research with Instructor Taymour Bibi [01:02:37] [Watch Now]
Find out more about this dynamic program and the emerging careers in the Clinical Research field.
*From 00:23:10 – Instructor Taymour Bibi speaks about the careers in Clinical Research - Opportunities and Challenges for Clinical Research Post COVID-19 [00:55:08] [Watch Now]
* From 00:19:25 – Instructor Miran Kenk speaks about COVID-19 fundamentally changing medicine in Canada and around the world, drastically altering how we conduct clinical research.