Certificate in Clinical Research
Certificate in Clinical Research
Become an essential member of the clinical research process by learning how to protect patient safety, ensure trial integrity, and manage adherence to research ethics, best practices and regulations.
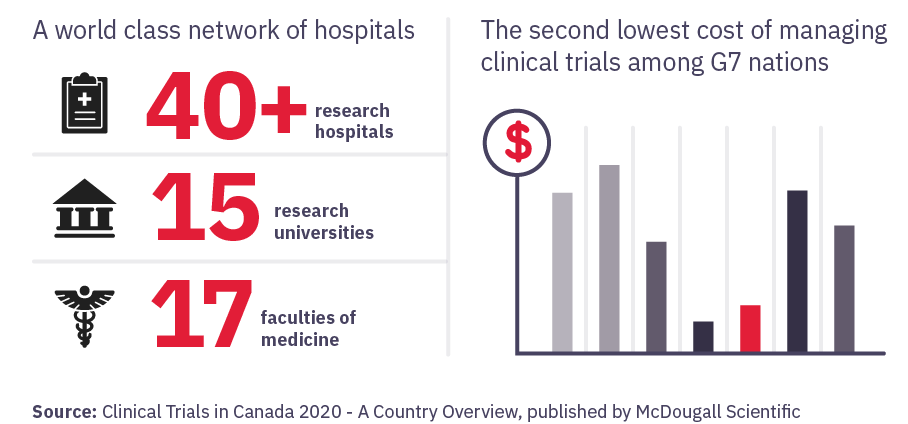
Canada is a world leader in clinical research
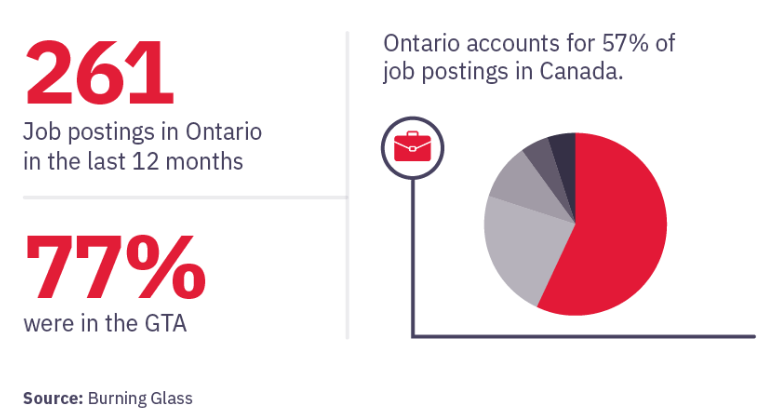
Canada currently ranks fourth in the world for number of clinical trial sites. The volume and growth of clinical trials taking place nationally signals promising job and career advancement opportunities.
What you will learn
- Understand the stages in setting up clinical trials
- Plan, manage, and monitor clinical research and trials
- Adhere to good clinical practice including patient consent, privacy, and data integrity protocols
- Abide by regulations and legislation to ensure that trials are conducted ethically, while upholding scientific research principles
- Demonstrate accuracy and reliability in data collection, management, and analysis
Program benefits
- Develop your skills through experiential assignments, projects, and case studies
- Learn from instructors who are leaders in the clinical research field
- Advance through the program with the same cohort of peers and build your professional network
- Participate in an applied clinical research management simulation capstone project
- Complete the program in 9 months through a combination of live classes and online learnings

Who Should Take This Program?
This program is designed for individuals with a health sciences background who are looking to enter this exciting field.
- New Graduates and Early Career Professionals with a related degree
- Internationally Educated Medical Doctors (IMDs) and Internationally Educated Health Practitioners (IEHPs)
- Trained Nurses (RNs, RPNs)
- Laboratory and Medical Technicians & Technologists
- Clinical Research professionals looking to upskill
Get Program Information
Enrolment Open

Program start date
Certificate in Clinical ResearchStarts: Sep 28, 2026
Ends: Jul 25, 2027